Based on a presentation at interphex in april 2019 1 this article discusses the contradictions between the us and eu requirements for cleanroom good manufacturing practices gmps.
Eu gmp clean room standards.
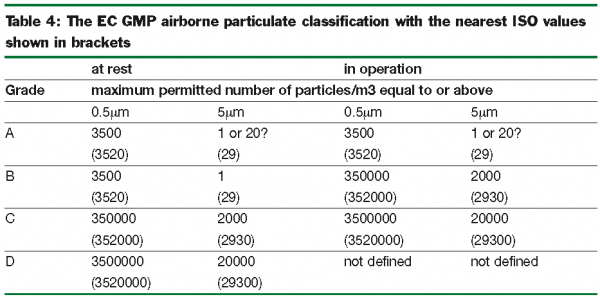
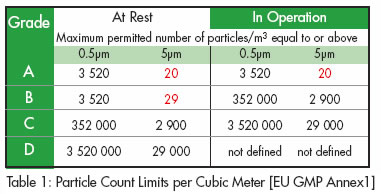
Maximum permitted number of particles per m 3 equal to or greater than the tabulated size.
1 cleanroom classification to iso 14644 1 2015 concentrates on the room performance not risk to the product.
Cleanrooms are designed to maintain extremely low levels of particulates such as dust airborne organisms or vaporized particles.
Annex 1 of both the eu and pic s guides to gmp and other standards and guidance as required by local health authorities.
Cleanroom practitioners have long held concerns about annex 1 because the classification of air cleanliness test methods and vocabulary are not harmonised with iso 14644.
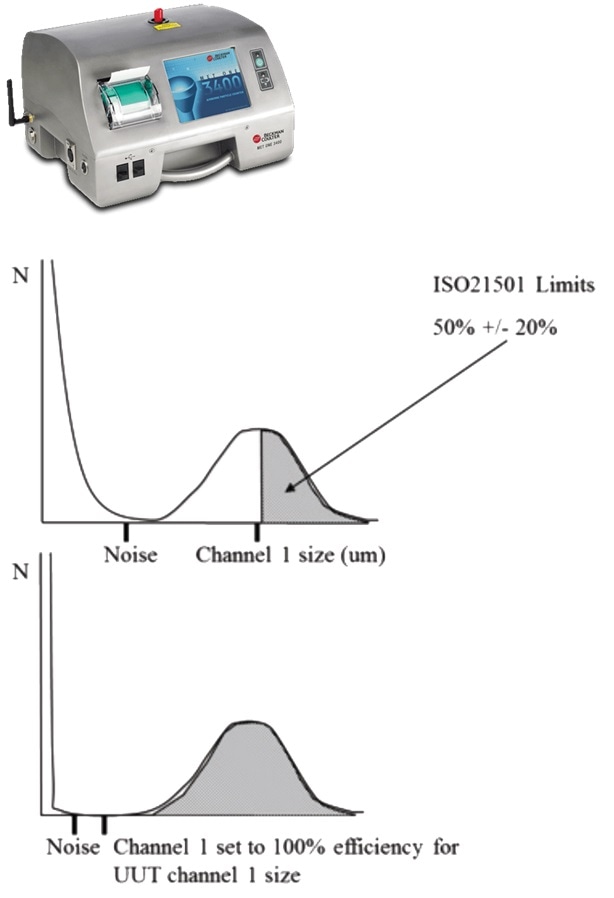
Both eu gmp annex 1 2009 1 and the fda cgmp 2004 2 state that classification is done to the method defined in iso 14644 1 2015 3 cgmp follows the maximum concentrations defined in iso 14644 1 2015 for each of the cleanroom grades.
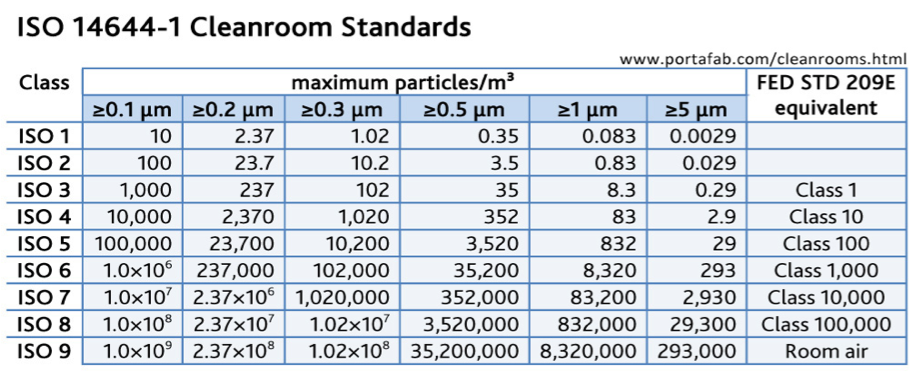
Discover the different classes within the cleanroom iso standards and their federal standard and gmp equivalent.
The basis of cleanroom standards is the micrometer or micron for short µm which is the size of the particles to be filtered.
Iso 14644 cleanroom classification table classes and requirements.
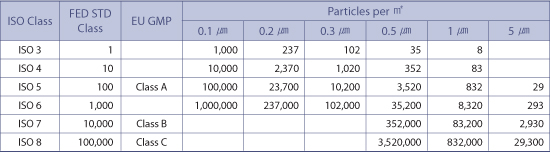
A b c and d are defined in the eudralex the rules governing medicinal products in the european union volume 4 eu guidelines to good manufacturing practice medicinal.
The european commission has implemented a set of standards for anyone who is located in europe and is involved in the manufacture of sterile products.
Manufacture of sterile medicinal products.
Routine monitoring risk analysis and interlocking doors.
The eu gmp pic s ich and who guidelines are referred to in dr hans schicht s regulatory reflections column in clean air and containment review.
A cleanroom or clean room is a facility ordinarily utilized as a part of specialized industrial production or scientific research including the manufacture of pharmaceutical items integrated circuits crt lcd oled and microled displays.
Cleanroom classification particle count.
These documents were previously restricted to uk nhs users.
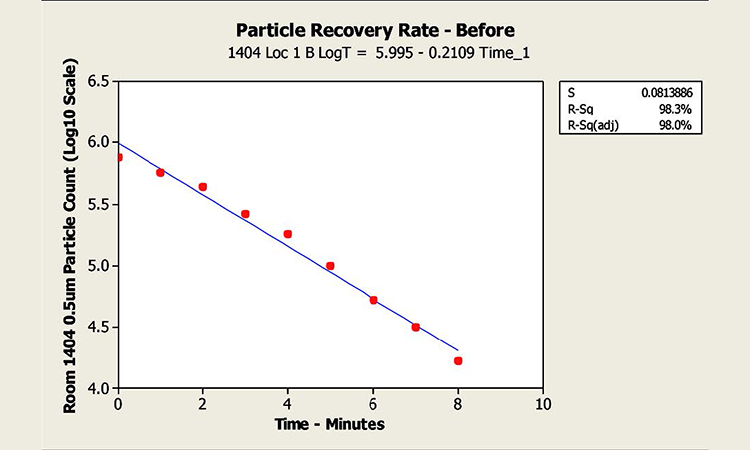
The author discusses particle concentration for cleanrooms at rest particle contamination in the air start up testing vs.
As stated before cleanrooms are classified by how clean the air is according to the quantity and size of particles per volume of air.
Ec gmp annex 1.
In a pharmaceutical sense clean rooms are those rooms that meet the code of gmp requirements as defined in the sterile code of gmp i e.